Every client, every project being unique, we tailor our solutions to fit your needs.
Our expertise : BioMechanics and high resolution imaging in Biology
All-inclusive skills in cellular biology, skin biology, physics, 3D modelisation of biological systems:
- Development of new imaging approaches and biophysics characterization
- Expertise in cellular and molecular biology, skin biology, immunology
- Statistics analyses and 3D reconstruction
- 3D design systems for maintaining biological samples
Our Open Lab and equipment dedicated to your challenges
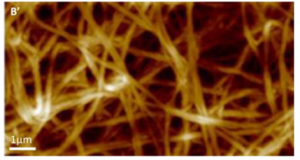
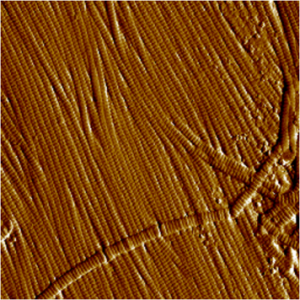
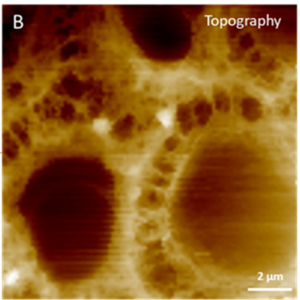
Atomic Force Microscopy (AFM)
Principle: This is a visualization technique based on the atomic scale interaction between a fine tip placed on a flexible cantilever and the examined sample. Scanning of the sample in an imaging mode yields the surface topography to be recorded with micrometric to nanometric resolution.
Advantages:
- Expertise in cellular and molecular biology, skin biology, immunology
- Real-time measurements
- Analyses, mechanical characteristics extraction and cartography (elastic modulus, firmness, viscoelasticity, adhesion, etc)
- Spectroscopy (proteins folding, molecular recognition, etc).
- Biomaterial and biotechnology (tissue engineering, pharmacological tests, etc).
Epifluorescence microscopy
Principle: Epifluorescence microscopy is an optical microscopy technique which relies on labeling the molecule of interest with a fluorescent probe. It consists of the excitation of the fluorescent molecule by a laser (excitation wavelength) which will allow it, returning to a stable state, to produce a photon (emission length) which will be captured by a sensor (camera).
This approach allows to visualize labeled molecules or structures in a biological sample.
Advantages: This system is coupled with our AFM system to localize areas to analyse by atomic force microscopy.
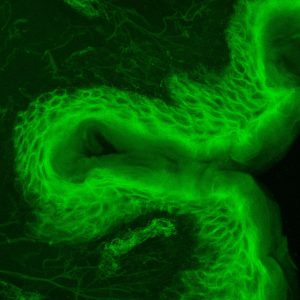
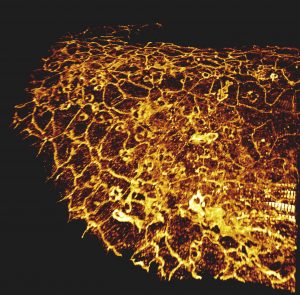
Confocal microscopy
Principle: Confocal microscopy is an optical microscopy technique to produce images with shallow depth of field, that is to say on a single focal plane about 300 to 400 nm thick using fluorescent probe.
By acquiring images of our sample on different focal planes, we obtain a three-dimensional reconstruction of it. Here the sample is scanned by the laser and the photons emitted are captured by a photomultiplier.
Advantages:
- Localisation of molecules labelled in multi-layer tissues
- Study of cellular organisation in multi-layer tissues
- Analyses of labelled molecules’ colocalisations
- Etc.
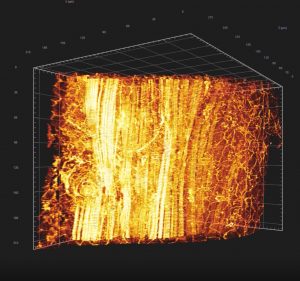
Second Generation Microscopy (SHG)
Principle: This technique uses a two-photons microscope coupled with a pulsed laser capable of emitting two photons of long wavelengths combining in a focal point. These long wavelengths are able to penetrate further into the sample to excite the targeted fluorophore.
Thanks to near infrared wavelengths for the incident light, TPEF has the ability to construct three-dimensional images of specimens by imaging deeper into thick tissues.
In addition, this technique allows us access to the generation of second harmonic light (SHG). SHG requires intense laser light passing through a material with a non-centrosymmetric molecular structure such as collagen, present in the dermis, or myosin, present in muscle fibers…
As SHG doesn’t require the excitation of molecules, we avoid the effects of phototoxicity or photobleaching.
Also, since many biological structures produce strong SHG signals, the labeling of molecules with exogenous probes is not necessary, and we can preserve the way a biological system functions.
Advantages: visualization and imaging of:
- collagens I IV
- myosin muscles
- elastin and other auto fluorescent molecules
- Etc.
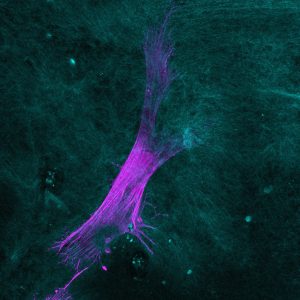
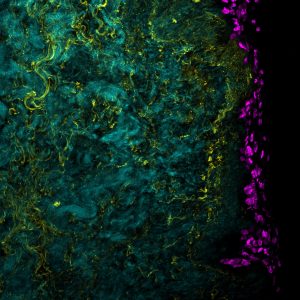
Light sheet fluorescence microscopy
Principle: Light sheet microscopy, like confocal microscopy, makes it possible to produce three-dimensional images from the fluorescence emitted by markers. However here the configuration of the microscope is fundamentally different. As a reminder, a confocal microscope is equipped with an objective which will allow the concentration of the light source on the sample but also the recovery of the signal. Here, we have a perpendicular configuration between the light source and the acquisition objective. In addition, it is our sample that will move (z + rotation).
The light source is adapted to provide complete illumination of the focal plane to be imaged. This illumination is called a light sheet. Some devices, such as the one we use, have two light sources placed on the same axis allowing uniform and optimal illumination of the sample.
The acquisition is not made by a photomultiplier but by a CCD camera (Charge Coupled Device) which allows to capture the entire focal plane very quickly.
The real advantage of this technique is to be able to apprehend large samples by the double illumination of the sample but also by the possibility of rotating the sample to have acquisitions from different angles and thus reconstruct it computationally.
Advantages:
- Thick tissues 3D analysis and reconstruction
- Phenotype illustration