All living cells depend on their mechanical surroundings. Their structure and function are directly linked to this mechanical stimulus (shear stress, gravity).
Cells sense these physical forces by their integrin-based adhesion (“outside-in” and “inside-out”) and translate them into biochemical and biological signals through mechanotransduction.
This ability to respond to physical environment changes is significant in the homeostasis of tissues constantly exposed to mechanical stress.
This response can modify cellular and extracellular structure (i.e., ECM*) by activating signalling pathways and then modulate diverse functions such as protein synthesis, proliferation, differentiation, or apoptosis. Thus, a dysfunctional cellular mechanotransduction leads to mechanobiological diseases. Many mechanobiology diseases are due to a disruption in the force transmission between the extracellular matrix (ECM), the cytoskeleton and the interior of the nucleus.
Biophysics, among other fields, have developed crucial tools (micro- or nano- sensors) allowing to measure and apply very small forces to investigate the interaction forces between cellular components, molecules inside cells and even the cell response to its environment.
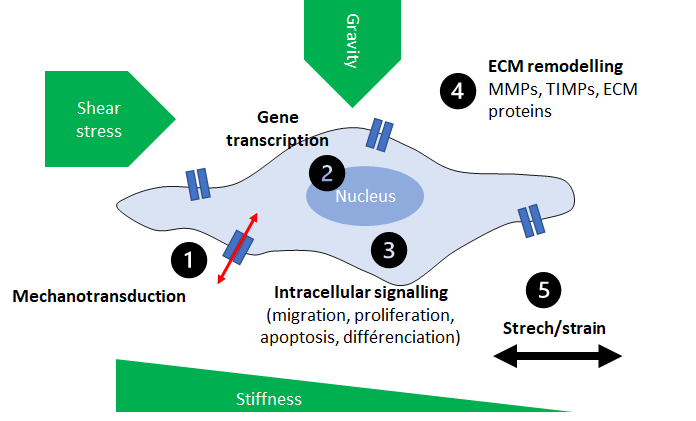
Mechanotranduction process is represented as a mechanobiological feedback loop of cells: 1) sensor, 2) translocation to nucleus, gene transcription, 3) intracellular signalling, 4) ECM remodelling by MMPs, TIMPs and ECM protein secretions and 5) cell shape by stretch/strain phenomenon. Cellular activities are controlled via this feedback loop such as proliferation, migration, differentiation, apoptosis and even protein secretion able to remodel ECM.
Atomic Force Microscopy (AFM) is one of the techniques which measures the mechanical properties of cells and tissues. AFM is able to both image and mechanically manipulate a biological structure near physiological environment over time. Thanks to a tip fixed at the end of a cantilever, AFM taps gently on the surface of the cell and the deflection of the cantilever gives information about the stiffness** of the region taped.
ECM stiffness has been widely studied in biomechanics and is defined by the amount of proteins present such as collagen, fibronectin or proteoglycans. The orientation and degree of cross-linking of those proteins are determining too.
ECM stiffness manages a wide range of intra- and intercellular processes :
- A stiff matrix promotes cell spreading, proliferation and migration
- A soft matrix enables cells to spread into deeper layers of the tissue
- The deformation of the matrix promotes changes in intracellular signaling pathways and thus their phenotype.
Understanding of the relationship between biochemical and mechanical processes is essential to control and maintain tissue homeostasis and cell regulation. Thanks to biomechanics and AFM through external mechanical stimuli, we can better figure out ways to study mechanotransduction and more generally mechanobiology.
* The extracellular matrix (ECM) is the non-cellular framework for tissues and organs, where cells live, proliferate, migrate, or modify their phenotype. The ECM contains extracellular macromolecules such as collagen, enzymes, glycoproteins, and growth factors that creates structural and biochemical shape.
** Stiffness is the reaction in stress while an object resists deformation. For biological samples, stiffness is the amount of force required to deform their environment.
Consult our study cases
Tissue stiffness
Tumor escape and extracellular matrix
Evaluation of production of extracellular matrix components
Binding proteins characterization
Follow our other news
The relationship between skin elasticity and viscoelasticity
 24 January 2024
24 January 2024Are you a smoker? Here’s how it affects your skin!
 29 November 2022
29 November 2022Why are simplicity and multifunctionality new trends in the beauty market?
 19 October 2022
19 October 2022